Which of the Following Indicates an Endothermic Reaction
The value of is positive for an endothermic process. Endothermic reactions are chemical processes in which the reactants absorb heat from the environment to be completed.

Endothermic Reaction Definition Equation Graph Examples
2 PCI3 1 O2 g OPCI3 1 DHrxn -3251 kJ.

. AB C D heat C. These reactions cause a cooling effect by lowering the temperature of the surrounding environment. 10Energy will flow from substance A to substance B.
As it dissolves the beaker feels cold. Solid ammonium nitrate is added to a beaker of water. Less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst.
13 Specific heat capacity. For example the radius of the sun is approximately 696000 kilometers while bacterial cells are as small as 19 10-4 millimeters. Give a reason for the answer 2 33 Draw a sketch graph of potential energy versus course of reaction for the reaction above.
This means an increase in temperature occurs with. Scientists have developed a new drug known to cure a previously incurable disease. When the products of a reaction are cooler than the reactants.
This indicates energy is released and the reaction is exothermic. Write a balanced nuclear reaction for the following. Enthalpy is the heat involved in a reaction.
Heating water over a flame to its boiling point. 3 Get Other questions on the subject. 234 Pa 4 He 91 2 When balancing the.
Heat is released Water is formed Salt is produced Temperature decreases 2. Whereas when energy is released by the molecules of a substance in a reaction then it is known as an exothermic reaction. AB C D heat C.
Correct answer - Question 7 of 10 Which of the following indicates an endothermic reaction. What best explains if the new drug is a compound or not. Activation energy Heat of reaction ΔH Reactants and products 3 34 Determine the empirical formula of butane gas if it consists of 8276 carbon and 1724.
Solution for Is the forward reaction endothermic or exothermic. 15 Energy stored in the bonds of a substance C H bonds contain a. Clearly indicate the following on the graph.
A B heat C D heat D. A substance that can be added to the reaction mixture to indicate the equivalence. What is revealed in the reaction CaOH2s Ca2aq 2OH-aq H -1671.
Another question on Chemistry. 4 NH3 g 5 02 g 4NO2 g 6H2O I DHrxn -11692 kJ 3D C. Which of the following is described by the equation H2O l heat H2O g.
C s 02 g Co2 g DHrxn 9405 kcal b. The value of is negative for an exothermic process. Calculate the value of ΔH for this reaction.
Cooling a mixture of air and kerosene vapor to obtain liquid kerosene. Which of the following represents a chemical change. - If the container of a reaction becomes colder during the reaction the reaction is endothermic Calculate deltaH o rxn for the reaction A 2B - 2C using the deltaH o f values given below 2c -.
Which of the following are examples of endothermic reactionsprocesses. Select all that apply the reaction is exothermic the reaction is endothermic the reactants lost. A B Heat -- C D What is enthalpy.
Express each number in an alternate form. Choose as many as apply a. Which of the following energy diagrams shows a concerted endothermic reaction.
Cutting a piece of paper with scissors. It is a compound because it will be able to cure a disease. Scientists often have to deal with numbers that are either very large or very small.
Burning a piece of notebook paper in the presence of oxygen. Also in endothermic reactions is one whose enthalpy value is positive that is the system absorbs heat from the environment ΔH 0. Which of the following reactions shows that the formation of SO2 releases 2968 kJmol.
Chemistry questions and answers. KJmol Toitwool adolorib919 reaction coordinate reaction coordinate reaction coordinate reaction coordinate yam 2692 iw noite91 i r tobas 16101 DA to ngiz adib919. The reaction produces heat.
7 Liquid Z C 353 Jg-C. Which of the following indicates that an endothermic reaction has occurred. Which of the following indicates an endothermic reaction.
Which of the following indicates that an endothermic reaction has occurred. When hydrogen gas and oxygen gas react to form water a burst of flames is observed. Solid sodium hydroxide pellets are added to a beaker of water.
A B heat C. In an exothermic reaction the bonding energy of the product is. 12q m C ΔT.
In endothermic reactions the products have more energy than the reagents this means that they do not occur exponentially. This also means that a decrease in temperature occurs with absorption of heat. Indicate whether each of the following reactions is endothermic or exothermic.
It becomes an endothermic reaction. Correct answer to the question Question 7 of 10 Which of the following indicates an endothermic reaction. Endothermic reactions are those that absorb energy in the form of heat.
For example water absorbs heat from a burner to be converted into vapour.

Endothermic Reaction Definition Equation Graph Examples

Endothermic Reaction Definition Equation Graph Examples

H Is A State Function Because E P V Are State Functions So It Depends Only On The Difference Betwee Chemistry Education Teaching Chemistry Science Chemistry

A Endothermic Exothermic Semichem11ib
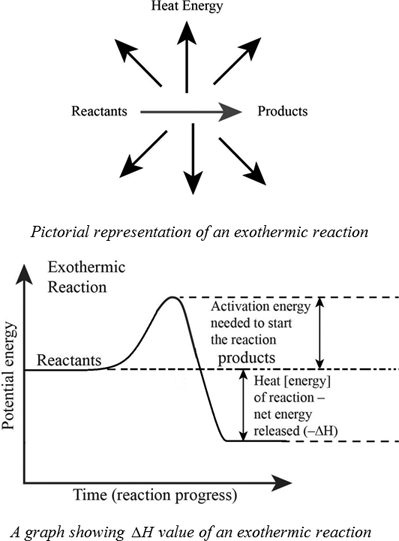
Definition Of Exothermic And Endothermic Reactions Chegg Com

Endothermic Exothermic Reactions Energy Changes In Chemical Reactions Mcat Content

Endothermic Vs Exothermic Reaction Graphs Youtube

Schematic Representation Of The Energy Level Diagram Of An Exothermic Download Scientific Diagram
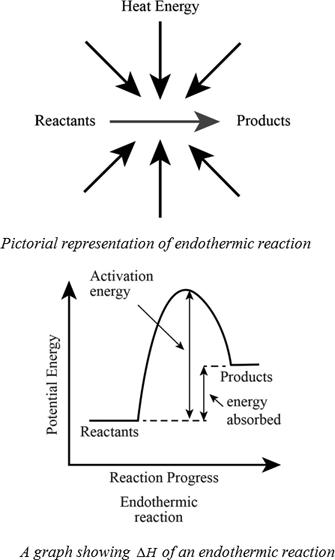
Definition Of Exothermic And Endothermic Reactions Chegg Com

Endothermic Reaction Definition Equation Graph Examples
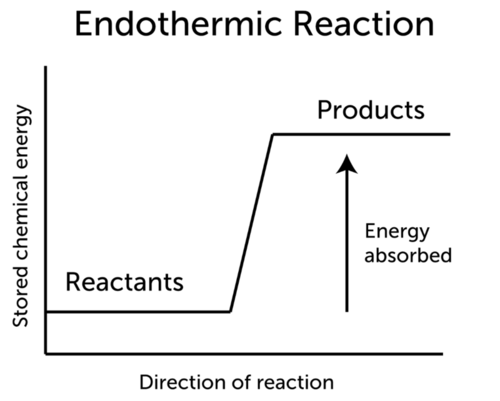
Endothermic Reaction Ck 12 Foundation

Endothermic Reaction Definition Equation Graph Examples

Endothermic Exothermic Reactions Energy Changes In Chemical Reactions Mcat Content

Heat Of Reaction Reflects The Difference In Enthalpy Between The Products And The Reactants Teaching Chemistry Chemistry Education Teaching Science

Exothermic And Endothermic Processes Chemistry For Non Majors

Endothermic Vs Exothermic Reactions Chemtalk

The Picture Above Is Showing An Example Of Endothermic Because It S Taking Energy From The Things Around It W Chemistry Physical Chemistry Exothermic Reaction

